News
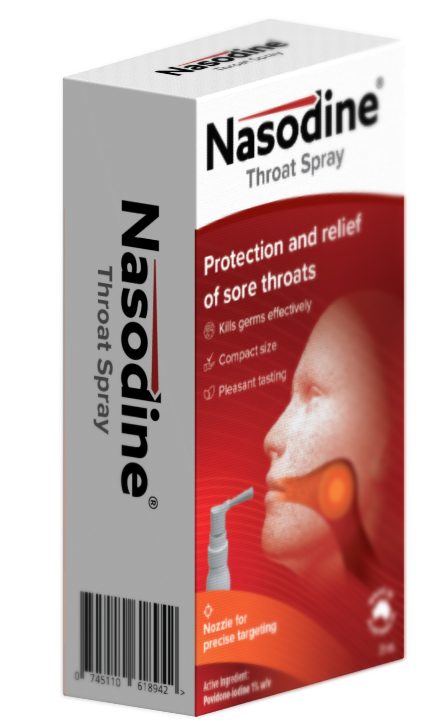
Firebrick announces new product: Nasodine® Throat Spray
Firebrick Pharma (ASX:FRE) is pleased to announce that it has commenced manufacture of Nasodine® Throat Spray for export in November 2025, which will be the first new product in the Nasodine range since Nasodine Nasal Spray, which was launched in Singapore in 2024.
Nasodine Throat Spray is a 1.0% povidone-iodine formulation for treatment of sore throats in a convenient throat spray form, as distinct from the throat gargle that is already available in many markets.
The low volume means there is no need to expectorate (spit out) unused solution, avoiding the potential staining and inconvenience of the gargle format. Details about Nasodine Throat Spray can be found in the full announcement.
Firebrick Pharma has received a permit from the TGA (Therapeutic Goods Administration) that allows export of the new Throat Spray product from Australia, but not local sale in Australia.
Firebrick has already placed on order for production of an initial batch of the Throat Spray with its Australian manufacturing partner, Probiotec.
Product is expected to be released and available for export during November 2025.
The first market where the Throat Spray will be launched is Singapore, where like Nasodine Nasal Spray, it can be sold as an antiseptic and does not require local regulatory approval.
The Company’s licensing partner, Innorini Life Sciences (Innorini), expects marketing of the Throat Spray to start in December, focused on Singapore hospitals and doctors (HCPs).
They expect the product to be available in the retail channel around mid-2026.
Nasodine Throat Spray is the first new Nasodine-brand product after Nasodine Nasal Spray and heralds a range of Nasodine-brand products in the future, which will also follow in the footsteps of the nasal spray.