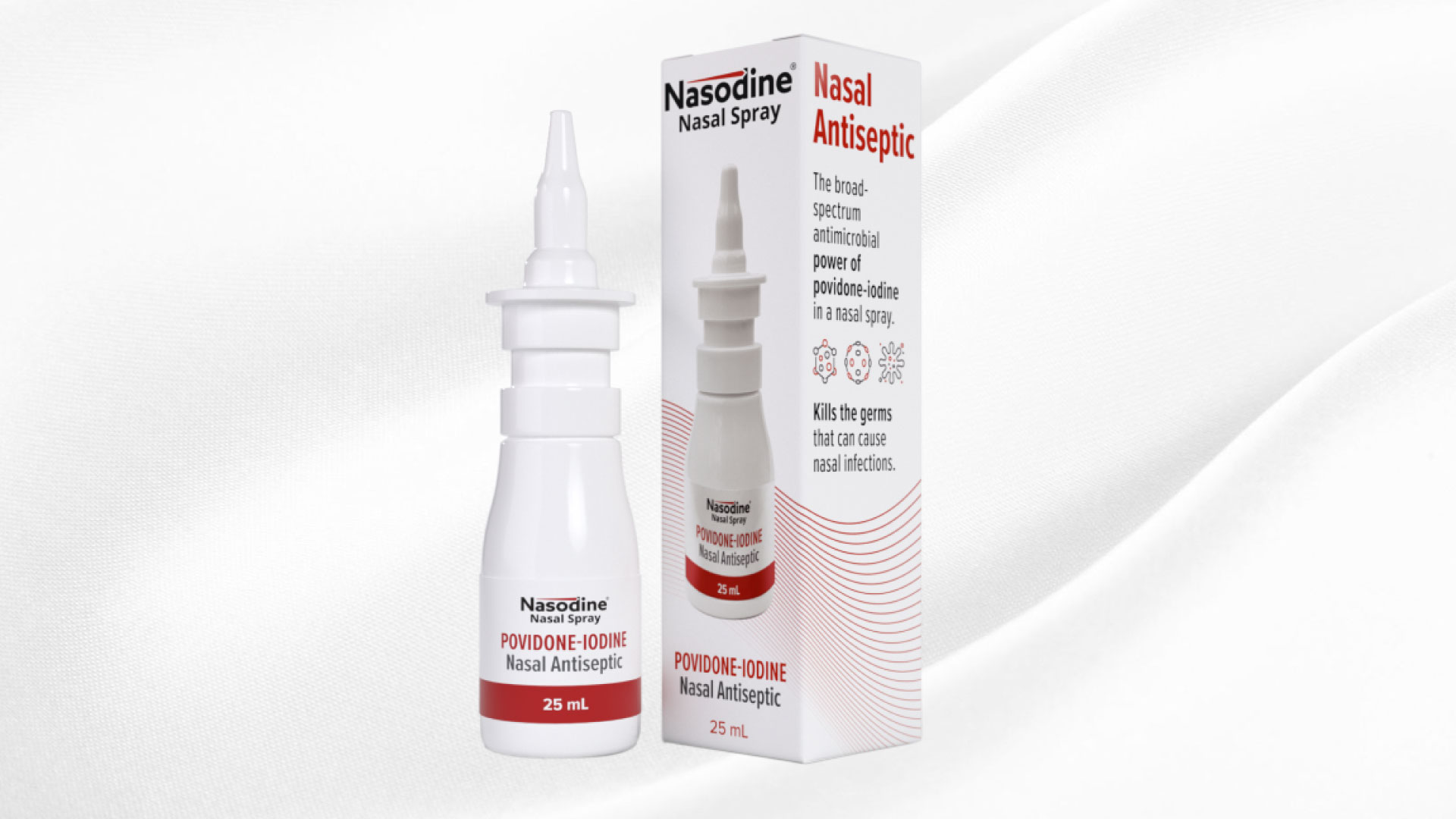
Posts Tagged ‘nasal spray’
Firebrick Launches Nasodine in Singapore via Nasodine-sg.com
Firebrick Pharma Limited (ASX:FRE) is proud to announce that its product, Nasodine® Nasal Spray, is now available for sale in Singapore. Customers will be able to order the product through Firebrick’s new website dedicated to support Singapore sales: www.nasodine-sg.com In Singapore, Nasodine is classified as a topical antiseptic and does not require approval or licensing…
Firebrick Pharma launches Nasodine in the US via Nasodine.com
Firebrick Pharma (ASX:FRE) has launched its nasal spray product, Nasodine® Nasal Spray in the United States, the first country in the world where the product has become commercially available.
Nasodine Phase 2 COVID-19 Trial Results Published
The Company is now advanced in our plans to introduce Nasodine in at least one international market.
Nasodine COVID-19 patent granted
HIGHLIGHTS • Patent now granted in US, Australia and South Africa • Patent supports proposed nasal disinfection use of Nasodine Firebrick Pharma is pleased to announce that our patent covering the use of Nasodine® Nasal Spray in COVID-19 has now been granted in South Africa. The patent has previously been granted in the US and Australia…
Nasal disinfection as a front-line defence in future pandemics
Recently, Professor Peter Friedland presented at the Australian Military Medical Association conference (12 – 15 October 2023), with the title of his presentation being: “Nasal Disinfection as a Front-line Defence in Future Pandemics”. Read the published abstract here To view the full presentation, please click here Disclosure: the presentation is the view of the presenter…
Update on Nasodine Trial and Forward Plans
Firebrick Pharma Limited announced that the independent preliminary investigation into the Phase 3 trial results of Nasodine Nasal Spray (“Nasodine”) (refer ASX announcement 13 September 2023) has not revealed any systematic error or other data issue that could explain or disclaim the reported results. The preliminary investigation phase has now been closed to avoid additional…
Firebrick Phase 3 Trial fully recruited with 500 subjects
Firebrick Pharma is pleased to announce that its Phase 3 trial of Nasodine® Nasal Spray in the treatment for the common cold has successfully completed recruitment, with 500 subjects enrolled. “Subject to availability of the complete efficacy data and timely completion of the statistical analysis, we expect to report headline results of the trial by…
Nasodine COVID-19 Trial Achieves Primary Endpoint
Highlights Firebrick Pharma Limited is pleased to announce that its Phase 2 trial of Nasodine® Nasal Spray in COVID-19 achieved its primary endpoint. The primary endpoint was the reduction in viral load of SARS-CoV-2 over 4 days, based on culturable virus from throat and nasal swabs. Nasodine treatment resulted in 100% reduction by day 4,…
Nasodine Phase 3 Clinical Trial Surpasses 80% Recruitment
Firebrick Pharma is pleased to provide an update on the progress of the confirmatory Phase 3 clinical trial of Nasodine® Nasal Spray as a treatment for the common cold. The study, which commenced in 2022, aims to recruit 196 subjects with early-stage colds who are confirmed by PCR to have a viral infection (other than…
Nasodine Patent Allowed in Canada
Highlights Firebrick Pharma Limited (ASX:FRE) is pleased to announce that its core patent covering Nasodine® Nasal Spray as a treatment and preventative for the common cold has now been allowed in Canada. Once this proceeds to grant, it will bring the number of countries where this patent has been granted to 28, now covering North…
Nasodine COVID-19 Trial Concludes Recruitment – Results in June 2023
Firebrick Pharma is pleased to announce that its Phase 2 COVID-19 trial of Nasodine® Nasal Spray has closed for recruitment.